Answer:
23.903 grams of product is produced
816.56grams of excess reactant was left over
Explanations:
The reaction between copper solid and sulfur is expressed as:
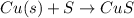
Determine the moles of copper
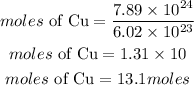
Determine the moles of sulfur
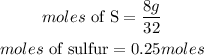
Since sulfur has less moles, hence sulfur is the limiting reactant
Calculate the mass of CuS
Mass = moles * molar mass
Mass of CuS = 0.25 * 95.611
Mass of CuS = 23.903 grams
Determine the mass of excess reactant consumed
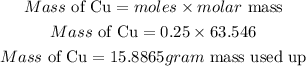
Mass of Cu started with = 13.1 * 63.546
Mass of Cu started with = 832.4526grams
Mass of excess reactant left over = 832.4526g - 15.8865g
Mass of excess reactant left over = 816.56grams
Therefore 816.56grams of excess reactant was left over