Answer:
0.946moles
Explanations:
Given the balanced chemical reaction shown below;
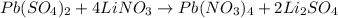
Given the following parameter
Mass of Lithium sulfate = 52 grams
Determine the moles of Lithium sulfate
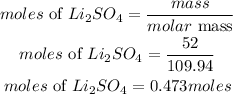
According to stoichiometry, 4moles of LiNO3 produces 2 moles of Lithium sulfate, the moles of LiNO3 required to produce this amount is given as;
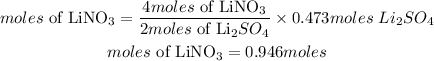
Hence the moles of LiNO3 are needed to produce 52 grams of Lithium sulfate is 0.946moles