
Given :-
- We have 10kg of water which is melting at the temperature of 50° C and 100° C
- The specific heat capacity of water is 4200J/Kg
- The specific latent heat of vaporisation of water is 2260 KJ/kg
Let's Begin :-
We have,
We know that,
Heat required for temperature change in the same phase
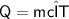
Subsitute the required values,
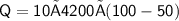
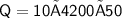
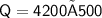
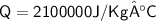

Now,
Heat required for phase change at the same temperature
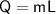
- L is the latent heat of vaporisation
Subsitute the required values,
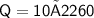
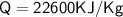
Therefore,
Total heat energy required to convert 10kg of water
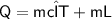
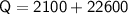

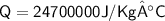
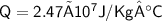
Hence, The heat required to convert completely 10kg of water at 50° C into steam 100° C is 2.47 × 10^7 J/kg°C or 2.47 × 10^4 kJ/kg°C.