First we need to know how to determine the molar mass and what variables we need to know. The molar mass is calculated as follows:
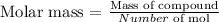
We already know the mass of our compound is equal to 1.30 g, but we need to know the number of moles present. We will determine them by applying the ideal gas equation:
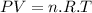
Where:
P = Pressure, atm
V= Volumen, L
n= Number of moles
R= Ideal constant gas
T= Temperature, K
Now, we clear n and we replace known terms:
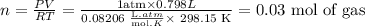
Now, we calculate the molar mass using the first question:
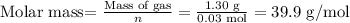
The molar mass of the gas will be 39.9 g/mol