In STP conditions, assuming the gas behaves as an Ideal gas, the volume of the gas and the number of moles of the gas are directly related, independet of the gas we are working with.
Since we are assuming we are using the old STP conditions, one mol is equivalent to 22.4 L, so we can use rule of three to find the answer:
n --- V
----------------------
1 mol --- 22.4 L
x --- 3.6 L
So, the relation is:
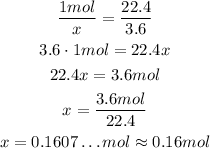
So, there is approximately 0.16 mol of the gas.