According to the given reaction, 2 moles of NO are produced.
Using the molecular mass of NO we can find the mass produced:
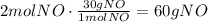
It means that when 60g of NO are produced the heat absorbed is 171KJ.
Use this ratio to find the heat absorbed when 2.3 grams are produced:
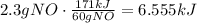
It means that the correct answer is 6.5kJ.