The question refers to the "volcano" reaction, which is a very common science experiment at school.
a) The "volcano reaction" consists in mixing baking soda (NaHCO3) with vinegar (CH3COOH) to produce carbon dioxide (CO2) and sodium acetate (CH3COONa).
The balanced reaction is:
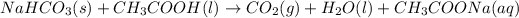
b) In this case, we don't have the exact amount used for both reactants, but we can say that sodium bicarbonate (NaHCO3) is the limiting reactant because vinegar was added in excess. Another way to see it is: vinegar was added just to completely react with the available amount of baking soda, which makes the baking soda the limiting reactant.
c) The actual mass of sodium acetate obtained can be calculated from the difference between the masses of flask+sodium acetate (125.24g) and just flask (122.60g):
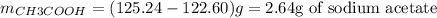
d) Considering the balanced reactin shown before and the amount of NaHCO3 used (4.15g), we can calculate the amount of sodium acetate that should be produced. But first, we need to know the amount of NaHCO3 used in moles (molar mass NaHCO3 = 84.00 g/mol):
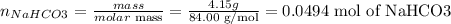
Now, we can calculate the amount of CH3COONa that should be produced and convert it to mass of CH3COONa (molar mass = 82.03 g/mol):
1 mol NaHCO3 ---------- 1 mol CH3COOH
0.0494 mol NaHCO3 ----- x
Solving for x, we have that 0.0494 moles of CH3COONa or 4.052 g of CH3COONa should be produced.
e) Since we know the amount of CH3COONa that should be produced (4.052g) and the amount actually obtained (2.640g), we can calculate the yield of the reaction:
4.052g of CH3COONa ---------- 100% yield
2.640g of CH3COONa ---------- y
Solving for y, we have that the reaction had an yield of 65.2%.