The question requires us to comment on the bonds that are formed when occurs the combustion of octane.
The combustion reaction of octane (C8H18) happens when this compound reacts with oxygen (O2) to form carbon dioxide (CO2) and water (H2O), according to the following chemical equation:
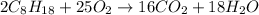
When this reaction happens, the covalent bonds between carbon (C) and hydrogen (H) in the octane molecule are broken and new bonds between cabon and oxygen atoms are formed to produce CO2. Similarly, hydrogens atoms "released" bond to oxygen atom to form H2O.
Therefore, the bonds C=O and H-O- are formed when octane is burned.