Step-by-step explanation:
First we have to find the total cost of the pencil pouch including taxes:
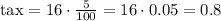
The tax is $0.80, so the total cost of the pencil pouch is $16.80.
The change is the difference between how much Alene pays and how much does the item cost:
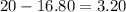
Answer:
She will receive $3.20 back