Answer:
0.03 L.
Step-by-step explanation:
What is given?
Volume 1 (V1) = 0.05 L.
Pressure 1 (P1) = 1.00 atm.
Pressure 2 (P2) = 1.45 atm.
What do we need? Volume 2 (V2).
Step-by-step solution:
To solve a problem when temperature is constant but volume and pressure vary, we use Boyle's Law. Boyle's law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases. The formula of this law is described by this:

We want to know the value of volume 2 (V2), so let's solve for this unknown value and replace the given data:
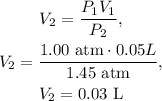
The final volume would be 0.03 L, as pressure increases, the volume decreases.