Answer:
Exothermic reaction. Option D is correct
Explanations:
Endothermic reaction is a reaction that occurs when the reactants of a chemical reaction absorbed energy from the surrounding to form the product while the exothermic reaction occur when the energy is released to the surroundings.
When natural gas (methane) is burned, energy is given off according to the equation;
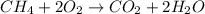
Since heat is given off, the chemical reaction that occur will be an exothermic reaction.