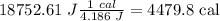Answer
Q=4479.8 cal
Procedure
To solve the problem you will need to use the specific heat formula
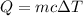
Where;
Q=heat energy
m=mass
c=specific heat capacity
ΔT=change in temperature
Assuming that the heat released from the cracker of unknown material is equal to the heat absorbed by the water, then we can use the c and m for water in our calculations.
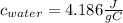
Substituting the values in our equation we have
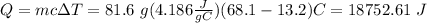
Finally, transform the J to cal