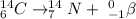Chemistry -> Nuclearchemistry -> Radioactivity
Beta decay is a kind of radioactive decay in which a beta particle is emitted from the atom.
A beta particle consists of only an electron that was ejected from the atom. Therefore, the notation for a beta particle is the following:
-1 indicates that there's only the electron and 0 for atomic mass indicates there are no protons or neutrons.
With the concept of a beta particle in mind, we can write the equation aiming to keep both sides of the equation balanced. For Carbon-14 (atomic number = 6), we have the following equation:
For the equation to be balanced in terms of atomic number and atomic mass, we simply do:
Atomic number
6 + 1 = 7
Atomic mass
and 14+0 = 14
The element that has an atomic number of 7 is Nitrogen (N). So the final equation is the following:
Final answer: