Given data:
* The value of the charge given is,
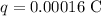
Solution:
Let n be the number of electrons in the given charge.
The net charge in terms of the number of electrons is,
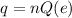
where Q(e) is the fundamental value of the charge,
Substituting the known values,
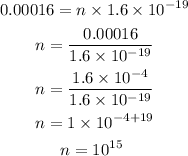
Thus, the number of electrons in the given charge is 10^(15).