What is an Acid?
An acid in terms of hydrogen is a solution that has positively charged hydrogen ions in the solution.
In this quesition, we are given some compounds which are acids and required to name the anion bonded to the acid,
HC₂H₃O₂
If we rewrite this compound, becomes CH₃COOH. This compound is called ethanoic acid. The cations and anions are
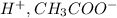
The anion bonded to the hydrogen atom is called an ethanoate.
H₂SO₄
This compound is called tetraoxosulphate(vi) acid. The cations and anions here are
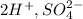
The name of the anion is called sulphate ion.
H₂SO₃
This compound is called trioxosulphate(v) ion and the cations and anions here are
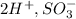
The name of the anion is sulphite ion
HClO₄
The name of the given compound is percholoric acid or chlorine oxoacid.
The cations and anions are
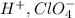
The name of the anion is perchlorate ion.