We will have the following:
First, we are given:
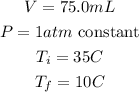
Now, we transform these units to the SI, that is:
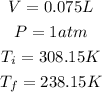
Now, using the initial values we determine the quantity of the the gas:
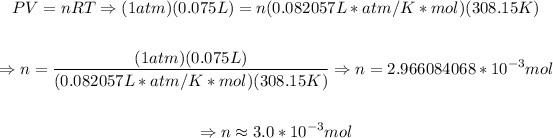
Now that we have the number of moles, then we determine the final volume, that is:
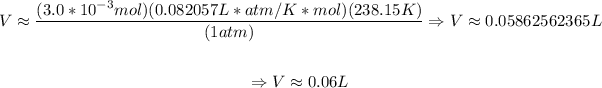
So, the final volume will be approximately 0.06 L.