The joules of heat released by the copper coin will be the same as the ones absorbed by the water.
To find the heat absorbed by the water can be found using the following formula:
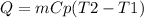
Where Q is the heat absorbed or released, m is the mass, Cp is the specific heat of the substance and T2 and T1 are the final and initial temperatures of the substance.
m can be found using the given volume of water and its density (1g/mL), Cp is 4.18J/g°C and T2 and T1 are given:
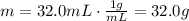
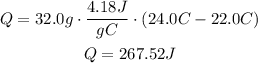
It means that the copper coin released 267.52 joules.