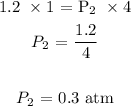Answer:
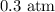
Step-by-step explanation:
Here, we want to get the final pressure of the gas
From Boyle's law, we know that the volume and pressure of a given mass of gas are inversely proportional at a constant temperature
Mathematically:
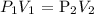
Where:
P1 is the initial pressure which is 1.2 atm
V1 is the initial volume which is 1.0 L
P2 is the final pressure which is what we want to calculate
V2 is the final volume which is 4.0 L
Substituting the values, we have it that: