1) Write the chemical equation
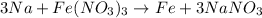
2) Which is the limiting reactant?
List the known quantities.
4.50 mol Na
2.25 mol Fe(NO3)3
How many moles of Fe(NO3)3 do we need to use all of the Na?
The molar ratio between Fe(NO3)3 and Na is 1 mol Fe(NO3)3: 3 mol Na.
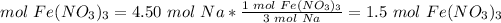
We need 1.5 mol Fe(NO3)3 and we have 2.25. We have enough Fe(NO3)3.
How many moles of Na do we need to use all of the Fe(NO3)3?
The molar ratio between Fe(NO3)3 and Na is 1 mol Fe(NO3)3: 3 mol Na.
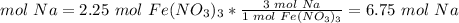
We need 6.75 mol Na and we have 4.50 mol Na. We do not have enough Na.
We need 1.5 mol Fe(NO3)3 and we have 2.25. We have enough Fe(NO3)3. This is the excess reactant.
We need 6.75 mol Na and we have 4.50 mol Na. We do not have enough Na. This is the limiting reactant.