The specific heat capacity is the heat required to raise the temperature of 1kg of a substance by 1 deg. celcius and is given by the following formula:
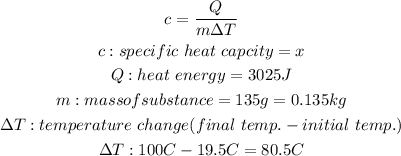
We will substitute these values into the equation to determine the unknown x:
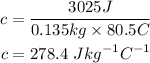
Answer: The specific heat of the metal is 278.4 J/kg deg. C.