To find the formula mass of tin(ii) chromate we multiply the subscript by the molar mass of that particulat element. If there are no subscript we multiply by 1.
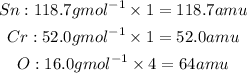
Total mass or formula mass of the compound is:
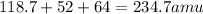
Dividing each element by the formula mass of the compound gives:
