The total internal energy U of a monoatomic ideal gas is given by:
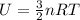
Where n is the amount of substance, R is the universal gas constant and T is the temperature of the sample. The value of R is:
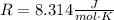
Replace n=1.3mol and T=315K to find the internal energy of the gas:
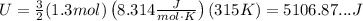
Therefore, the approximate total internal energy of the gas is 5100J. The correct choice is Option B.