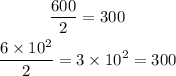We can use the relation between moles and number of molecules to find out how many hydrogen gas molecules (H2) were produced:
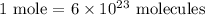
In this case, 0.5 moles of H2 were produced, so we can set the following proportion:
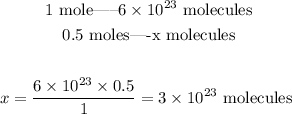
So, we have produced 3x10^23 molecules of hydrogen gas.