Simplify the equation to obain the value of x.
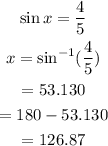
So value of x is 126.87, for x lies in second quadrant.
Determine the value of sin 2x.
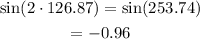
Determine the value of cos 2x.
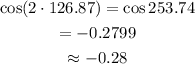
Determine the value of tan 2x.
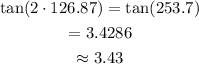
By using formula:
In second quadrant cos and tan have negative values.
Determine the value of sin 2x by using formula.
![\begin{gathered} \sin 2x=2\sin x\cos x \\ =2\sin x\cdot(-\sqrt[]{1-\sin ^2x}) \\ =-2\cdot(4)/(5)\cdot\sqrt[]{1-((4)/(5))^2} \\ =-(8)/(5)\cdot\sqrt[]{(25-16)/(25)} \\ =-(8)/(5)\cdot(3)/(5) \\ =-(24)/(25) \\ =-0.96 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/khooegiivsy5olcfq7u0dniv5uozl8oygv.png)
Determine the value of cos 2x by using formula.
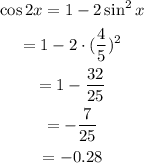
Determine the value of tan 2x by using formula.
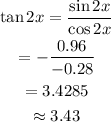
So values of the expressions are,
sin 2x = -0.96
cos 2x = -0.28
tan 2x = 3.43