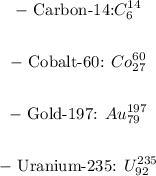Answer and explanation:
The nuclides with the same atomic number, but different mass numbers are isotopes.
So, in this case, each species will have the same atomic number and a different mass number, that will be the same that it is written in the name of the element: