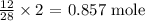Answer:
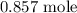
Step-by-step explanation:
Here, we want to get the number of moles of ammonia that can be produced
From the question, we have it theoretically that 1 mole of nitrogen gave 2 moles of ammonia
To get the number of moles of ammonia produced actually, we need to get the number of moles of nitrogen
To have this, we divide the mass of nitrogen molecule by its molar mass
The molar mass is 28g/mol
The number of moles is thus:
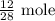
Since 1 mole of nitrogen gave 2 moles of ammonia
The number of moles of ammonia will be: