Step 1 - Understanding how pressure and temperature are related
There are three main variable that may modify the state of a gas: pressure (P), volume (V) and temperature (T). They are all related by the following formula:
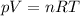
When one of these variables is kept constant (in this exercise, for example, volume is constant), linear relationships arise between the remaining variables.
So, at constant volume, presure and temperature become directly proportional to each other, which can be mathematically stated as:
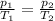
We can use this relation to solve the exercise.
Step 2 - Solving the exercise
According to the exercise:
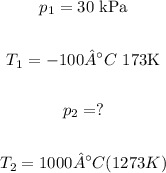
For this relation to work, the temperature must be used in K, not °C.
Setting the values in the equation, we get:
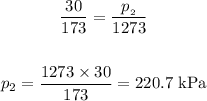
Answer: the final pressure in the tank is 220.7 kPa.