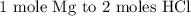Answer:
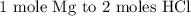
Step-by-step explanation:
Here, we want to get the correct mole-to-mole factor
From the balanced equation of reaction, 1 mole of magnesium solid reacts with 2 moles of hydrochloric acid
Hence, the mole to mole factor according to the balanced equation given is