The balnaced equation is:
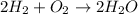
so 2 mol of H2 react with 1 mol of O2 and give 2 mol of water. Now we have to transform mols into mass. For that we use the molar mass of each molecule
Mm of H2 is 2g/mol
Mm of O2 is 32g/mol
and Mm of water is 18 g/mol
To pass the molar basis reaction into mass based we just have multiply the stechiometric factor by the molar mass of that molecule:
for Hydrogen:
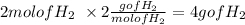
Oxygen:
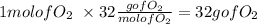
Water:
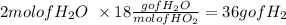
so now in mass based proportions the reaction can be expresed as 4 grams of H2 react with 32 grams of O2 to give 36 grams of water.
To answer the questions we need to find which is the limiting reactant, H2 or O2. To do that we calculate how much O2 is requiried to react with 5 grams of H2 knowin that we need 32 g of O2 for 4 g of H2. we use this as a conversion factor:
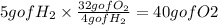
So to consume 5 grams of H2 we need at least 40 g of O2 and we only have 10 grams of O2. this means the limiting reactos is Oxygen.
For question B:
as we have said 32 grams of O2 produce 36 grams of water using conversion factors:
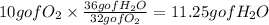
so 11.25 grams of water are produced
Part C.
We need to calculate first how much H2 reacts so we can calculate how much H2 is left
We know the we need 32 grams of O2 to consume 4 H2, we using this as a conversion factor:
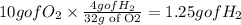
If only 1.25 grams of H2 are consumed of the initial 4 grams we have left :
4 g - 1.25 g= 2.75 g of H2