ANSWER
Potassium will contribute one electron to the sea delocalized electrons
Step-by-step explanation
Potassium is a group one metal and it has an atomic number is 19.
The electronic configuration of potassium is given below
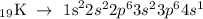
In the configuration above, potassium has one electron at it outermost shell. This implies that the valence electron of potassium is 1
Potassium is readily to lose one electron to to attain it octet structure
Therefore, potassium will contribute one electron to the sea delocalized electrons