We want to find the P2, given
V1 = 77 mL
V2 = 16 mL
T2 = 24 degrees celsius = 24 + 273 = 297 K
At standard conditions we know that temperature = 0 degrees celsius and pressure = 1 atm so:
T1 = o degrees celsius = 0 + 273 = 273 K
P1 = 1 atm
P2 = ?
To solve this problem, we will use the following equation:
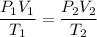
P2 = (1 atm x 77 mL x 297 K)/(273 K x 16 mL)
P2 = 5.235576923 atm