Answer:
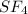
Step-by-step explanation:
For the octet rule, we know that there should be 8 atoms around the central atom
For SF4, sulfur has 6 valence electrons. If we factor in one electron by each of the fluorine atoms, we would have a total of 10 electrons
In that case, the octet rule will no longer be obeyed