We will call y to the Two Below price and x to the retail price.
We know that y (Two Below price) is always $2 below x (retail price).
Then, we can write the relation as:
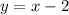
This will be the equation of the line relating both prices.
Answer: B. y = x-2.