The first step to answer this question is to balance the given equation, to do it assign 2 as the coefficients of NaOH and H2O:
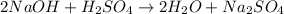
8. To find the number of moles used to neutralize the river water we have to multiply the volume used by the concentration of the NaOH solution, remember that this volume has to be converted to liters first and that M=mol/L:
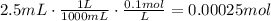
There are 0.00025moles of NaOH in the solution.
9. According to the given reaction, 2 moles of NaOH react with 1 mole of H2SO4. Using this ratio we can find the number of moles of sulfuric acid in the river:
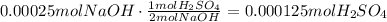
There are 0.000125moles of H2SO4 in the river.
10. To find the concentration in the river, divide the amount of moles by the volume of the river in liters:
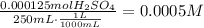
The concentration of H2SO4 in the river is 0.0005M
11. For every mole of H2SO4, there are 2 moles of H+, it means that the concentration of H+ ions is:
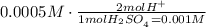
The concentration of the H+ ions in the river is 0.001M
12. Use the given formula to find the pH of the solution:
![\begin{gathered} pH=-log\lbrack H^+] \\ pH=-log(0.001M) \\ pH=3 \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/d0mcke1b1jqqxwzmzt1inm9q0u4mt58vda.png)
The pH of the river solution is 3.
13. Fish can tolerate neutral values of pH, those are within the range of 6.5-7.5. It means that these fish can't tolerate the acidity of this river.