Given: Principal amount of P= $618 compounded semi-annually for
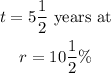
Required: To find the accumulated amount.
Explanation: The amount after t years at a rate of r% compounded semi-annually is
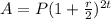
Here,
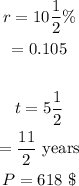
Putting these values gives
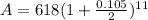
Solving this gives the value
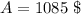
Final Answer: The accumulated amount is $1085.