The reaction we have is an endothermic reaction, since the deltaHrxn has a positive value and is equal to 1207kJ, that is, the reaction requires energy for it to happen.
The reaction equation is balanced and the energy value they give us corresponds to the number of moles that are in the reaction. If the value of moles changes the energy required will also change. Therefore, we must first calculate the moles of Cl2 that are formed.
To calculate the moles equivalent to 212.7g of Cl2 we will use its molar mass, equal to 70.9g/mol. Moles of Cl2 will be:
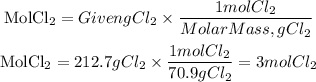
We have 3 moles of Cl2 that are formed. The energy given is for the formation of 6 moles of Cl2. To calculate the energy for 3 moles of Cl2 we can apply a rule of three:
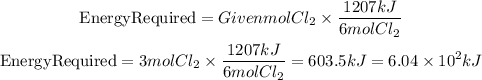
The energy required is 6.04x10^2kJ. The answer will be the third option.