Gay Lussac's law relates the pressure and temperature of a ideal gas in two different states at constant volume. In this case, they present us with a single state, they give us the grams of water vapor, so to find the volume we apply the ideal gas law. The ideal gas law has the following equation:
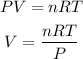
Where,
V is the volume of the gas
n is the number of moles,

R is a constant, 0.08206atm.L/mol.K
T is the temperature, at STP the temperature is 273.15K
P is the pressure, at STP the pressure is 1 atm
We replace the known data and find the volume:
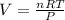
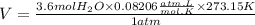
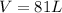
Answer: The volume of 65 grams of water at STP is 81 Liters