4.7 days
1) Gathering the data
45gram
K = 0.1474
2) To find out the substance half-life, we have to plug into that formula the following data for the amount of mass
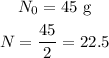
Since the half-life is the time a substance gets to half of its initial amount. So we can write out, remembering that e= 2.718:
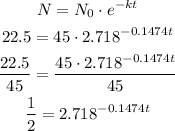
Now we can apply the logarithms to both sides:
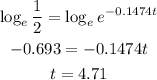
Hence, the answer is the half life is 4.7 days (rounded off to the nearest tenth)