The equation of the reaction that we are given is balanced, so we can continue with the calculations.
We first find the moles that are equivalent to 5.7 grams of KI. For that, we divide the grams by the molar mass of the compound. The molar mass of KI is 166.0028g/mol.
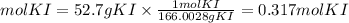
Now, we find the moles of KI taking into account that the ratio Pb(NO3)2 is 1/2. The moles of KI will be:
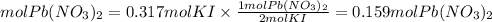
We multiply the moles of Pb(NO3)2 by the molar mass equal to331.2g/mol.
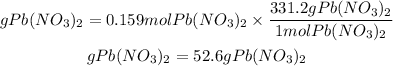
answer: The mass of Pb(NO3)2 needed to react is 52.6 grams