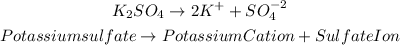Dissociation in chemistry is a general process in which complexes, molecules, or salts are separated into smaller molecules, ions, or radicals, usually in a reversible manner.
For hydrochloric acid (HCl) we will have the following dissociation equation
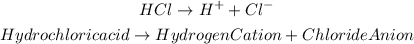
For the salt, potassium sulfate we will have: