Answer:
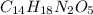
Explanations:
Given the following parameters
Carbon = 57.14%
Hydrogen = 6.12%
Nitrogen = 9.52%
Oxygen = 27.2%
Convert the percentages to grams
Carbon = 57.14g
Hydrogen = 6.12g
Nitrogen = 9.52g
Oxygen = 27.2g
Convert the mass to moles
Mole of carbon = 57.14g/12 = 4.76moles
Mole of hydrogen = 6.12g/1= 6.12moles
Mole of nitrogen = 9.52/14 = 0.68 moles
Mole of oxygen = 27.2/16 = 1.7moles
Divide by the lowest number of moles
For Carbon: 4.76/0.68 = 7(2) = 14
For hydrogen: 6.12/0.68 = 9(2) = 18
For nitrogen: 0.68/0.68 = 1(2) = 2
For oxygen: 1.7/0.68 = 2.5(2) = 5
Determine the empirical formula
Empirical formula = C14H18N2O5
Determine the molecular formula
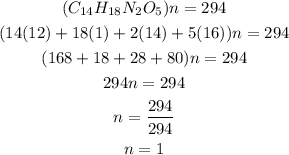
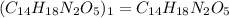
Hence the molecular formula of aspartame is C14H18N2O5