To answer this question, we have to use the combined law of gases, that states that:
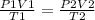
Where P1 and P2 are the initial and final pressures, V1 and V2 are the initial and final volumes and T1 and T2 are the initial and final temperatures. In this case, P1, V1, T1, P2 and T2 are given and we have to find V2:
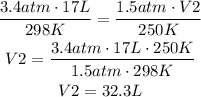
It means that the new volume of the gas is 32.3 L, the correct answer is C.