Molarity is the measure of how many moles has a liter of solution.
It means that it can be used as a conversion factor to find how many liters of solutions are needed.
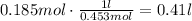
Convert the amount of liters to milliliters:
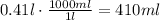
410ml of the solutions are needed for the required amount of NaNO₃.