i) In order to determine the absolute error, take into account that you have a set of measurement, that is, you calculate the mean absolute error.
Use the following formula:
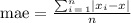
where xi are the different measures and x is the expected value. In this case
x = 3.92.
Replace the given data into the previous expression and simplify:
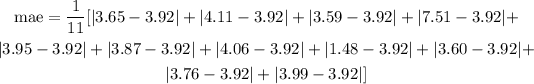
After simplification you obtain:
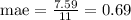
Hence, the mean absolute error is 0.96