Answer:
The final concentration is 0.45mol/L.
Step-by-step explanation:
1st) It is necessary to calculate the moles of iron (II) nitrate contained in 35mL of 0.15 mol/L solution:
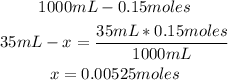
In this solution there are 0.00525 moles of iron (II) nitrate.
2nd) Now we have to calculate the moles contained in the 72mL of 0.60 mol/L solution:
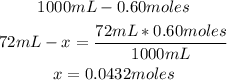
In this solution there are 0.0432 moles of iron (II) nitrate.
3rd) Finally, we have to add both amounts of moles (0.00525 moles + 0.0432moles = 0.04845 moles) considering the total volume (35mL + 72mL = 107mL):
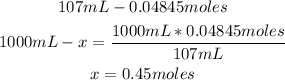
So, the final concentration is 0.45mol/L.