The pressure exerted on an object immersed on a fluid is given by:
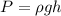
where rho is the density, g is the gravitational acceleration and h is the depth or height of the object in the fluid.
The denisty of sea water is 1026 kg/m^3 and we know the depth in this case is 24 m, then we have:
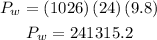
Hence the pressure in water is 241315.2 Pa.
For the air we know the density is 0.870 kg/m^3, the height is 7.5 km, then we have:
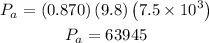
Now, that we know this we can calculate the change in pressure:
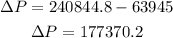
Therefore, the change in pressure is 177370.2 Pa.