Step 1
Gay-Lussac's Law states that the pressure exerted by a gas (of a given mass and kept at constant volume) varies directly with the absolute temperature of the gas.
Mathematically:
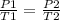
P1 = pressure 1
T1 = absolute temperature 1
---
P2 = pressure 2
T2 = absolute temperature 2
-----------------------
Step 2
Information provided:
P1 = 12 atm
T1 = 25 °C + 273 = 298 K
----
P2 = unknown
T2 = 75 °C + 273 = 348 K
------------------------
Step 3
From step 1, P2 is found as follows:
P1/T1 = P2/T2
(P1/T1) x T2 = P2
(12 atm/298 K) x 348 K = 14 atm
Answer: P2 = 14 atm