Answer:
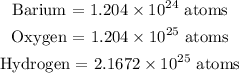
Step-by-step explanation:
Here, we want to know the number of atoms of each of the elements present in the given term
For the Barium, it is only affected by the external 2, so we have 2 Barium atoms only
For oxygen, we have a set in OH and another in H2O. For the OH own, we have 2 oxygen atoms and for the H2O , we have 8 atoms. That makes a total of 10, which when multiplied by the first 2 outside, gives 20
For Hydrogen, we have two sets, one with OH and the other with H2O
For the one with OH, we have 2 while for the one with H2O, we have 16. That makes a total of 18 which when multiplied by 2 gives a total of 36
Thus, we have each of the elements and their counts as follows:
Barium = 2
Oxygen = 20
Hydrogen = 36
Mathematically:
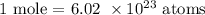
Thus:
