The given reaction is exothermic, we know igt because of the sign of the change in enthalpy given by the question, which is negative.
Exothermic reactions have negative changes in enthalpy.
The change in enthalpy can be found by multiplying the given amount of moles of aluminium by the given change in enthalpy:
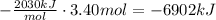
The change in enthalpy is -6902kJ.