The given value of the number of the moles is,
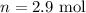
The value of the temperature is,
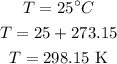
The value of the pressure is,
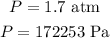
By using the ideal gas equation,
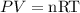
where R is the gas constant.
The value of the gas constant is,
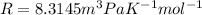
Substituting all the known values,
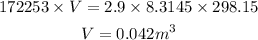
Thus, the value of the volume occupied by the oxygen gas is 0.042 meter cube.